
Graft Survival Affects Hair Transplant Result
Importance Of Graft Survival
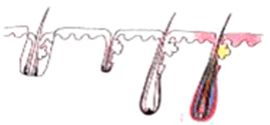
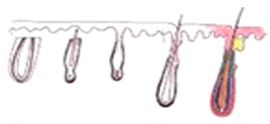
After a person is born, there will not be an additional hair follicle. The permanent hair follicles at the back of our head, the donor site, are invaluable and cannot be replaced. Many people expect hair follicles to be cloned, but the date of success is an unknown. The priority is to minimize the trauma of hair follicles during the process of hair transplantation. Over the past 50 years, many studies have been conducted to look at the actual survival rate of transplanted hair, and the percentage growth, reporting the following findings :
• Hair is considered an “organ” for transplantation
• Like other organs, they must be carefully stored after leaving the body, otherwise they will die
• Damaged hair follicles may not produce new hair after transplantation
• Generally requires at least 85% of transplanted hair to grow to see result
• The causes of hair follicle damage can be divided into " H-Factor " and " X-Factor "
Graft Survival and Donor Scar
When more grafts survive, less is required to complete the job. As width of the FUT donor scar is proportional to the width of the strip excised, a higher survival will reduce the donor scar.
Graft Survival and Final Result
The final result of the hair transplant procedure depends on how many grafts actually regrow. Dr Beehner commented that the percentage of graft survival depends on the skill of the surgical team more than anything. In another word, the skill and experience of the surgical team is one of the most important factors in arriving at satisfactory result. The doctor and the staff should not just focus on extraction, but also on how to keep it growing after transplanting into another site. Taking out a graft cannot guarantee its growth unless the H-Factors and X-Factors are looked into. We therefore saw the need to evolve from UR-FUT into FUT-X.
Graft Survival and Future Sessions
Hair transplant is challenged by the limited donor supply. Until now Hair Cloning is still not available. The best way to ensure adequate supply of hair follicles for the next procedure is to minimize graft wastage in the first session.
Cost of Procedure
When more grafts survive, less is required to complete the job. As most hair transplant centers charge "per graft transplanted" not "per graft survives", a higher survival will bring the cost down. Having say that, money cannot buy back a wastage of good donor hair follicles.
Center A |
Center B |
|
|---|---|---|
Cost per Graft (HKD) |
25 |
25 |
| No of Graft Required | 2,400 | 2,400 |
| Total Cost (HKD) | 60,000 | 60,000 |
| Transection Rate (Graft damage) | 20% | 2% |
| No. of Damage Graft | 2,400 X 20% = 480 | 2,400 X 2% = 48 |
| No. of Intact Graft | 2,400 - 480 = 1,920 | 2,400 - 48 = 2,352 |
| Actual Cost per Graft (HKD) | 60,000 ÷ 1,920 = 31 | 60,000 ÷ 2,352 = 25.5 |
| CONCLUSION : A high transection rate increases the cost per graft. | ||
H-Factor and X-Factor
Transplanting a graft from one site to another does not guarantee good result. As damaged graft cannot re-grow, the surgeon must pay attention to every step and every details of the procedure. On average 85-92% of grafts survive after transplant and grow new hair. 2 possible ways a graft can be damaged or even killed - the X-Killing Factor and the H-Killing Factor.
"H" stands for human. Poor growth is caused by a breach of protocol. H-factor may occur before, during, and after the procedure. The result of a hair transplant procedure cannot therefore be guaranteed.
What Kills A Graft - X Factor

"X" stands for unknown. The term was first described by Dr Shield (Australia) in 1984, when the reduced hair growth was unexpected and unexplained. In early years this X-Factor only accounts for a very small 0.5-1% of all no growth. With the advance and standardization of surgicial techniques, X-Factors now accounts for the majority of unsatisfactory results.
Possible X Factors
It is of our opionion that such "unknown" factors are in fact biological damage to the grafts which takes plce during and after the procedure, including:
• Denied or missed H-Factors
• Reperfusion Ischaemic Injury
• Auto-rejection of Grafts
• Overheating and Dehydration of Grafts in the First Week
• Improper haircare or activities
What Kills A Graft - H Factor

H-Factors Before Procedure
Predisposing Factors That Compromise Result include:
• Poor Circulation of the Recipient Area
• Scar on to-be-transplanted areas
• Smoking
• Uncontrolled Diabetes
H- Factors During Procedure
Grafts are injured During Procedure:
• Dehydration
• Excessive Removal of Supporting Tissue
• Cold Injury
• Transecting the Hair Follicles
• Chemical Trauma To Grafts During Storage
• Ischaemic-Reperfusion Injury
H-Factors After Procedure
Transplanted Follicles are injured after the procedure
• Failure To Compile With Instruction
• Failure to Form New Circulation
• Physical Trauma To Transplanted Graft
• Infection
How We Improve Graft Survival

H Factor 1 - Dehydration
Dehydration causes serious graft damage. Deleterious changes in cell integrity when follicles are left to dry for more than 5 minutes (Gandelman). When grafts are exposed to air for more than 10 minutes, more than 6% will die. After 20 minutes survival markedly decreases (Kim)
Duration of air exposure (min) |
Percentage of Growth |
|---|---|
0 |
96 % |
| 5 | 94 % |
| 10 | 94 % |
| 20 | 83 % |
| 30 | 63 % |
Dehydration causes serious graft damage. Deleterious changes in cell integrity when follicles are left to dry for more than 5 minutes (Gandelman). When grafts are exposed to air for more than 10 minutes, more than 6% will die. After 20 minutes survival markedly decreases (Kim)
How We Overcome Dehydration
• Do not leave grafts on the gloved fingers
• Avoid using FUE for more than 2,500 grafts ( 5,000 Hairs )
• Avoid using implanters
• All grafts are submerged in Hypothermosol solution or moistened gauze pads
H Factor 2 - Excessive Removal of Stem Cells
Removing too much tissue around the hair follicle is also a form of physical injury. Stem cells responsible for follicular re-growth is located at the tissue surrounding the graft. Seager reported in 1997 that Chubby Graft (more fat) survives better than Skinny Graft (less fat) (Seager). In 2010 Beehner commented that the differences in survival is that the stem cells are retained in chubby grafts but trimmed away in the skinny grafts
% growth after 19 months |
Skinny Graft |
Chubby Graft |
|---|---|---|
2-hair follicular units |
69.3 % |
88.0 % |
| 1-hair follicular Units | 48 % | 98 % |
How We Overcome Excessive Stem Cells Removal
• Use microscopes to preserve stem cell containing tissues
• When using FUE for harvesting always use a larger punch to retain the fatty tissue
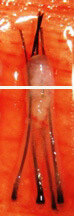
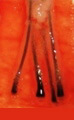
H Factor 3 - Graft Transection
Transected the follicle at upper 1/3 |
||
 |
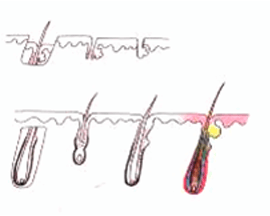 |
Upper 1/3 - 0% will grow |
| Lower 2/3 - 83% will grow | ||
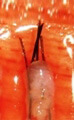
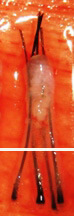
Transected the follicle at the middle |
||
  |
  |
Upper 1/2 - 40% will grow |
| Lower 1/2 - 27% will grow | ||
Transected the follicle at lower 1/3 |
||
 |
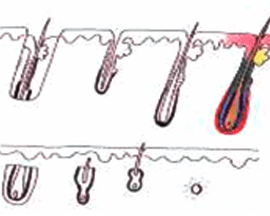 |
Upper 2/3 - 65% will grow |
| Lower 1/3 - 0% will grow | ||
Graft transection can be reduced to less than 2% overall rate by cutting the graft under direct and magnified vision.
How We Reduce Transection 1 : Open Technique
Blind harvesting technique is still commonly used in strip excision. Patient sits and leans forward. The surgeon stands behind and cut the strip without seeing the hair follicles. Those experienced adjust blade angles to follow the existing hair. This may work for Caucasian's short roots, but not for Asians' longer ones. Follicle are transected on the course of the blind blade. More blades are used, lesser grafts survive.
This technique was first introduced by Dr Pathomvanich in 1998, Open Donor Harvesting has not enjoyed popularity. It's time-consuming taking an extra 30 minutes, thus used mainly by surgeons who care about scar and final result.
How We Reduce Transection 2 : Microscopic Dissection
In FUT the smallest follicular unit is used for grafting. Important parts of the follicle can easily be transected during cutting. Such grafts will yield suboptimal growth even if survived. The only way to preserve integrity of the follicles is to dissect under magnification.
The use of microscope was first introduced by Dr Limmer (USA) in 1991. However it took 10 years to become the gold standard. We are convinced that the result well worth the investment in equipment and training. We routinely use 10X stereoscopic microscopes with back lighting for better visualization. Cool LED light is used for illumination to avoid over-heat. Grafts are kept moist all the time to prevent dehydration. We have seven assistants for graft cutting grafts to shorten preparation time. Stem-cell containing tissue preserved for better survival.
H Factor 4 - Delay Re-implantation
| Duration After Extraction (hour) | % of Growth |
|---|---|
2 |
95 |
| 4 | 90 |
| 6 | 86 |
| 8 | 86 |
| 24 | 79 |
From the table you can see that graft survival to 90% after 4 hours. So after 4 hours 10% of the extracted grafts will not grow hair. This is a wastage as the donor hair is limited in a life time.
How We shorten the Dealy
• Limited FUE Extraction time to 2-3 hours
• Well planned procedure to shorten the operating time
• A large surgical team to share the work load
• Only perform one case a day so that all attention will be given
H Factor 5 - Cold Injury
This is a form of physical injury. Study had shown that all frozen graft will die. Domestic-grade refrigerator may have temperature fluctuation. If the fridge temperature is set too low it may drop below 0°C and kill grafts without knowing. From studies there was no significant difference in survival within 6 hours, whether the grafts were stored in room temperature or 4 °C (Kim)
How We Overcome cold Injury
• Store graft at room temperature if procedure can be complated within 4 6 hours
• Use a special storage solution ( Hypothermosol ) to store grafts in the fridge
• Try to complete implantation within 6 hours
H Factor 6 - Damage To Grafts During Storage
When tissue is removed from the body, the cells will slowly run out of oxygen and eventually die
Our Protocol
• Use ATP as Storgae Solution
• Shorten Surgical time
H Factor 7 - Ischaemic-Reperfusion Injury
After a period of low oxygen, when the grafts are implanted and suddenly re-exposed to oxygen, they may form free radicals. These free radicals may cause cell injury and suboptimal growth, and is known as "Ischaemic Reperfusion Injury or IRI" on Re-implanted Graft.
How We Overcome IRI
• Suppress tissue injury by the use of anti-inflammatory medication
• ATP is added to the storage solution to protect the grafts
H Factor 8 - Infection
Infection if recognized and treated promptly should not affect the final result.
Our Infection Control Protocol
• Use of antibiotics for selected case
• Perform procedure in an aseptic envirnoment
• All staff has to follow protocol
• Follow-ups should be conducted by experienced staff to look for early sign of infection
H Factor 9 - Physical Trauma Transplanted Graft
The first week after transplant the grafts can be dislodged by direct impact, especially when the patient fails to compile with instruction
Our Aftercare Protocol
• Provide detail postop instruction
• Free doctor follow-up. Arrange same day appointment for any immediate concern.
• A helpful team to answer any of your concern
• A specially designed cap is provided to protect the transplanted areas
H Factor 10 - Failure to Form New Circulation
Failure to form new circulation may starve the follicles and retard their growth
Our Protocol to improve circulation
• Request patient to reduce smoking
• Immediately use Low Laser Level Therapy and ATP Spray after the procedure
ATP energy delivery solution (USA)
What is ATP ?
ATP (Adenosine triphosphate) is an energy molecule that catalyzes a variety of important cellular reactions in our bodies. The lack of ATP can cause cell damage and eventual cell and tissue death. Because the creation and regulation of ATP requires oxygen, tissues deprived of oxygen often experience ATP loss and become damaged. Because of this, ATP becomes crucial for patients healing from wounds; especially surgical wounds.
In 2002, Dr. William Ehringer a Professor at the University of Louisville, School of Medicine, discovered a method to encapsulate and deliver ATP to cells. After 10 years of research the final formulations were identified - Lyposomal ATP. In 2012, Dr. Ehringer founded Energy Delivery Solutions in manufacturing ATP for use in post-surgical patients, patients with delayed wound healing, and in hair restoration.
We Use ATP During and After Hair Transplant
Experiments by Dr Cooley and Dr Pathomvanich our Medical Advisor showed that, in hair transplant surgery, Lyposomal ATP allows for quicker healing and better hair transplant yields. In 2015 we met with Dr Ehringer founder of Energy Delivery Solutions. Dr Ehringer and Dr Cooley both recommended:
1. Store grafts in ATP-containing storage solution
2. Spray ATP Formulation at home onto the recipient scalp first 48 hours, up to 5 days
Clinical trials on selected subjects for over a year revealed very impressive result. Since October 2016 we provide Lyposomal ATP to all patients routinely. As Lyposomal ATP has only a very short half life, we have to import from the state every few weeks. The benefit is well worth the extra efforts and expenses.
Low Level Laser Therapy

Hair transplants can initially traumatize the scalp and can result in a temporary hair loss during the first 4 months (this is known as shock loss). Some patients may experience swelling in the transplanted area. The transplanted donor follicles can also experience difficulty adapting to their new environment. Clinical studies have demonstrated the following beneficial effects of laser when use in conjunction with hair transplant.
• minimizes hair shedding (shock loss)
• strengthen hair follicles after surgery with a much higher probability of survival
• reduce swelling, redness and inflammation post-surgery
Effect of Laser in Cell Energy Supply
Laser hair therapy stimulates the mitochondria in cells to increase the production of adenosine triphosphate (ATP). ATP is the form of energy used by hair cells to grow imto follicles. Abundant energy supply is critical when dealing with weakened and traumatized hair follicles.
Increase Success Rate after Hair Transplant
Laser hair therapy devices have been used by thousands of hair transplant centers all over the world (such as Bosley and HairClub). However handheld contraptions made with cheap Light Emitting Diodes (LEDs) are worthless when it comes to energizing the base of hair follicles. Technologically advanced device with the FDA-cleared is now available in our center for use after hair transplant. A 20 minutes of treatment is able to revive the mitochondria of hair cells. This can result in stronger hair follicles with a higher probability of surviving the operation. These extra amounts of “survivor” hair grafts will eventually grow into healthy, terminal hairs.
Read More
References
• Aby Mathew. A review of Cellular Bippreservation Consideration During Hair Transplantation. Hair Transplant Forum International, Vol 23 No. 1, January/February 2013
• Baust, J.G. In: J.G. Baust and J.M. Baust, eds. Advances in Biopreservation. CRC Press, 2007; 1-14.
• Snyder, K.K., et al. Biological packaging for the global cell and tissue therapy markets. BioProcessing Journal. 2004(May/June); 1-7.
• Reyes, A.B., J.S. Pendergast, and S. Yamazaki. Mammalian peripheral circadian oscillators are temperature compensated. J Biol Rhythms. 2008; 23:95-98.
• Allen, F.M. Physical and toxic factors in shock. Archives of Surgery. 1939; 38:155-180.
• Bigelow, W.G., W.K. Lindsay, and W.F. Greenwood. Hypothermia: its possible role in cardiac surgery: an investigation of factors governing survival in dogs at low body temperature. Annals of Surgery. 1950; 132:849-866.
• Swan, H., et al. Hypothermia in surgery: analysis of 100 clinical cases. Annals of Surgery. 1955; 142:382-400.
• Collins, G.M., M. Bravo-Shugarman, and Pl. Terasaki. Kidney preservation for transplantation: initial perfusion and 30 hours ice storage. Lancet. 1969; 2:1219.
• Taylor, M.J., et al. A new solution for life without blood: asanguineous low flow perfusion of a whole-body perfusate during 3 hours of cardiac arrest and profound hypothermia. Circulation. 1995; 91:431-444.
• Van Buskirk, R.G., et al. Assessment of hypothermic storage of normal human epidermal keratinocytes (NHEK) using Alamar Blue. In Vitro Toxicology. 1996; 9:297-303.
• Mathew, A.J., J.G. Baust, and R.G. Van Buskirk. Optimization of HypoThermosol for the hypothermic storage of cardiac cells—addition of EDTA. In Vitro Toxicology. 1997; 10(4):407-415.
• Mathew, A.J., et al. Vitamin E and EDTA improve the efficacy of HypoThermosol—implication of apoptosis. In Vitro Toxicology. 1999; 12(3):163-172.
• Dahdah, N.S., et al. Effects of HypoThermosol, an experimental acellular solution for tissue preservation and cardiopulmonary bypass, on isolated newborn lamb coronary vessels subjected to ultraprofound hypothermia and anoxia. Cryobiology. 1999; 39:58-68.
• Mathew, A.J., J.G. Baust, and R.G. Van Buskirk. Improved hypothermic preservation of human renal cells through suppression of both apoptosis and necrosis. Cell Preservation Technology. 2003; 1:239-254.
• Baust, J.M., et al. Transplantation diagnostics: utilization of protein microarray analysis to determine kidney status and transplantation efficacy. Cell Preservation Technology. 2004; 2(2):81.
• Mathew, A.J., et al. Cell preservation in reparative and regenerative medicine: evolution of individualized solution composition. Tissue Engineering. 2004; 10:1662-1671.
• Snyder, K.K., et al. Biological packaging for the global cell and tissue therapy markets. BioProcessing Journal. 2004; 3(3):39.
• Van Buskirk, R.G., et al. Hypothermic storage and cryopreservation: the issues of successful short-term and long term preservation of cells and tissues. BioProcess Int’l. 2004; 2(10):42.
• Baust, J.M. Advances in media for cryopreservation and storage. BioProcess International. 2005; 3:46-56.
• Snyder, K.K., et al. Enhanced hypothermic storage of neonatal cardiomyocytes. Cell Preservation Technology. 2005; 3(1):61-74.
• Mathew, A.J. I’m losing cell viability and function at different points in my process, and I don’t know why! BioProcess Int’l. 2010; 8(6) 54-7.
• Ikonomovic, M., et al. Ultraprofound cerebral hypothermia and blood substitution with an acellular synthetic solution maintains neuronal viability in rat hippocampus. CryoLetters. 2001; 22:19-26.
• Lodish, H.F., et al. Transport across cell membranes. In: S. Tenney, ed. Molecular Cell Biology. 4th Edition. New York: W.H. Freeman, 2000; 590-591.
• MacKnight, A.D.C., and A. Leaf. Regulation of cellular volume. Physiology Review. 1977; 57:510.
• Martin, D.R., et al. Primary cause of unsuccessful liver and heart preservation: cold sensitivity of the ATPase system. Annals of Surgery. 1972; 175:11.
• Boutilier, R.G. Mechanisms of cell survival in hypoxia and hypothermia. J of Experimental Biology. 2001; 204:3171-3181.
• Toledo-Pereyra, L.H., A.J. Paez-Rollys, and J.M. PalmaVargas. Science of organ preservation. In: L.H. ToledoPereyra, ed. Organ Procurement and Preservation for Transplantation. Landes Bioscience, 1997; 1-16.
• Rehncrona, S., B.K. Siesjo, and D.S. Smith. Reversible ischemia of the brain: biochemical factors influencing restitution. ActaPhysioliogy Scandinavia. 1979; Suppl 492:135.
• Cotterill, P. ISHRS survey majority using chilled saline. W. Reed Personal communication, December 2012. Reed, W. Personal communication, December 2012.
• Perez-Meza, D., M. Leavitt, and M. Mayer. The growth factors. Part 1: clinical and histological evaluation of the wound healing and revascularization of the hair graft after hair transplant surgery. Hair Transplant Forum Int’l. 2007; 17(5):173.
• Perez-Meza D. Wound healing and revascularization of the hair transplant graft: role of the growth factors. In: W. Unger and R. Shapiro, eds. Hair Transplantation, 4th Ed. Marcel Dekker, New York, 2004; 287-294.
• Cooley, J. Ischemia-reperfusion injury and graft storage solutions. Hair Transplant Forum Int’l. 2004; 14(4):121,127,130.
• Parsley, W.M., and D. Perez-Meza. Review of factors affecting the growth and survival of follicular grafts. J Cutan Aesthet Surg. 2010; 3(2):69-75.
• Limmer, R. Micrograft survival. In: D. Stough, ed. Hair Replacement. Mosby Press, 1996; 147-149.
• Kim, J.C., and S. Hwang. The effects of dehydration, preservation temperature and time, and hydrogen peroxide on hair grafts. In: W.P. Unger and R. Shapiro, eds. Hair Transplantation, 4th Ed. New York: Marcel Dekker, 2004; 285-286.
• Hwang, S.J., et al. The effects of dehydration, preservation temperature and time on the hair grafts. Annals of Dermatology. 2002; 14:149-152. 37. Krugluger, W., et al. Enhancement of in vitro hair shaft
elongation in follicles stored in buffers that prevent follicle cell apoptosis. Dermatol Surg. 2004; 30:1-5.
• Krugluger, W., et al. New storage buffers for micrografts enhance graft survival and clinical outcome in hair restoration surgery. Hair Transplant Forum Int’l. 2003; 13(3):333-334.
• Van Buskirk, R.G., et al. Navigating the post-preservation viability fog: assay standardization for cell and tissue therapy applications. Genetic Engineering News. 2006; 26(19):38-39.
• Van Buskirk, R. Viability and functional assays used to assess preservation efficacy: the multiple endpoint/tier approach. In: J.G. Baust and J.M. Baust, eds. Advances in Biopreservation. CRC Press-Taylor and Francis Publishing: New York, 2006.
• Belzer, F., and J. Southard. Principles of solid-organ preservation by cold storage. Transplantation. 1988; 45(4):673-676.
• Taylor, M.J. The role of pH and buffer capacity in the recovery of function of smooth muscle cooled to –13°C in unfrozen media. Cryobiology. 1982; 19:585-60.
• Taylor, M.J., and Y. Pignat. Practical acid dissociation constants, temperature coefficients and buffer capacities for some biological buffers in solutions containing dimethyl sulfoxide between 25 and –12°C. Cryobiology. 1982; 19:99-109.
• Fujita, J. Cold shock response in mammalian cells. J Mol Microbiol Biotechnol. 1999; 1(2):243-255.
• Plesnila, N., et al. Effect of hypothermia on the volume of rat glial cells. Journal of Physiology. 2000; 523.1:155-162.
• Tseng, Y-C, et al. Exploring uncoupling proteins and antioxidant mechanisms under acute cold exposure in brains of fish. PLoS ONE. 2011; 6(3):1-15.
• Corwin, W.L., et al. The unfolded protein response in human corneal endothelial cells following hypothermic storage: Implications of a novel stress pathway. Cryobiology. 2011; 63(1):46-55.
• Baust, J.M. Overview of hypothermic storage. Hair Transplant Forum Int’l. 2006; 16(2):53.
• Cosentino, L.M., et al. Preliminary report: evaluation of storage conditions and cryococktails during peripheral blood mononuclear cell cryopreservation. Cell Preservation Technology. 2007; 5:189-204.
• Baust, J.M., R.G. Van Buskirk, and J.G. Baust. Cell viability improves following inhibition of cryopreservation-induced apoptosis. In Vitro Cell and Developmental Biology. 2000; 36:262.
• Baust, J.M., et al. A molecular basis of cryopreservation failure and its modulation to improve cell survival. Cell Transplantation. 2001; 10:561.
• Baust, J.M., R.G. Van Buskirk, and J.G. Baust. Gene activation of the apoptotic caspase cascade following cryogenic storage. Cell Preservation Technology. 2002; 1:63.
• Baust, J.M. Molecular mechanisms of cellular demise associated with cryopreservation failure. Cell Preservation Technology. 2002; 1:17.
• Anderson, R.V., M.G. Siegman, and R.S. Balaban. Hyperglycemia increases cerebral intracellular acidosis during circulatory arrest. Annals of Thoracic Surgery. 1992; 54:1126-1130.
• Ely, S.W., and R.M. Berne. Protective effects of adenosine in myocardial ischemia. Circulation. 1992; 85:893-904.
• Bessems, M., et al. Preservation of rat livers by cold storage: a comparison between the University of Wisconsin solution and HypoThermosol. Ann Transplant. 2004; 9(2):35-37.
• Povsic, T., et al. A double-blind, randomized, controlled, multicenter study to assess the safety and cardiovascular effects of skeletal myoblast implantation by catheter delivery in patients with chronic heart failure after myocardial infarction. American Heart Journal. 2011; 162(4):654-662.
• Powell, R., et al. Interim analysis results from the RESTORE-CLI, a randomized, double-blind multicenter phase II trial comparing expanded autologous bone marrow-derived tissue repair cells and placebo in patients with critical limb ischemia. J of Vasc Surg.2011; 54(4):1032-1041.
• Ginis, I., B. Grinblat, and M. Shirvan. Evaluation of bone marrow-derived mesenchymal stem cells after cryopreservation and hypothermic storage in clinically safe medium. Tissue Engineering Part C Methods. 2012; 18(6):453-463.
• Lowe, N.J., P.L. Lowe, and J. St Clair Roberts. A phase IIa open-label dose-escalation pilot study using allogeneic human dermal fibroblasts for nasolabial folds. Dermatol Surg. 2010;
36(10):1578-1585.
• Raposio, E., et al. Effects of cooling micrografts in hair transplantation surgery. Dermatol Surg. 1999; 25:705-707.
• Jiange, Q., et al. How long can hair follicle units be preserved
at 0 and 4°C for delayed transplant? Dermatol Surg. 2005; 31:23-26.
• Kurata, S., et al. Viability of isolated single hair follicles preserved at 4°C. Dermatol Surg. 1999; 25:26-29.
• Beehner, M.L. Notes from the Editor Emeritus. Hair Transplant Forum Int’l. 2005; 15(6):193-195.
• Cooley, J.E. Successful extended storage of hair follicles using hypothermic media with liposomal ATP. Translational Regenerative Medicine Forum. 2010.
• Beehner, M.L. 96-hour study of FU graft “out-of-body” survival comparing saline to HypoThermosol/ATP solution. Hair Transplant Forum Int’l. 2011; 21(2):1, 37.
• Ehringer, W.D. New horizons in storage solutions and additive agents in organ transplantation. Norwood Lecture, ISHRS 2011 Annual Scientific Meeting.

