

免責聲明 本文並不針對任何一款合成纖維絲,任何併發症乃施術者而非生產商的責任。
不建議人工植髮的三大原因
1. FDA 及 ISHRS 自 1983 年禁用
2. 風險甚高,併發症持續出現、難以處理,甚至不懂得如何處理;以後或查不能再做植髮。
3. 看不到有任何好處,特別是女士天生額高,及男士雄禿 II-V 期; 且效果不持久,容易脫落,日常生活極受限制!
被美國 FDA 封禁 40 年的人工植髮
所為人工植髮 Synthetic Hair Implant, 用的是 Prothetic Hair Fibres 合成纖維絲,代替自生的有生命的健康毛囊,植入頭皮的禿頭區域。因為這些纖維絲的根部沒有毛囊,醫生要使用鉤子扣在頭皮最深層的頭骨膜上,以防止拉扯過度脫落,所以過程比正統植髮祗植入到較淺的真皮層,更具入侵性。因為植的不是真髮,所以不能稱為植髮。早在 1983 年美國 FDA 已全國禁此 Prothetic Hair Fibres 作醫療產品使用,至今仍未解禁。
禁制原因
從 1978 年 12 月到 1981 年 2 月,FDA 收到了 166 宗有關人工植髮的投訴。 其中包括感染、臉部腫脹、劇烈疼痛、疤痕和剩餘真髮永久性脫落的病例,許多病例需要廣泛的醫療和手術治療。 FDA 官員表示,還可能有長期罹患癌症的風險。 其中七宗案例,患者必須透過手術切除部分頭皮。 在 21 例中,纖維絲無法全部去除,患者的頭皮永久性毀容。
1983 年,FDA 根據 《醫療器材禁令條例》 禁止使用該纖維,原因如下:
1. 由於纖維的非生物相容性 ( Biocompatibility ) 和植入物的非醫療性能 ( Non-medical performance ),纖維存在致病或創傷的風險。
2. 人工植髮屬欺詐行為,原因如下:
a) 誇大功效的欺騙性資訊;
b) 植入風險的隱瞞;
c) 沒有顯示對公眾健康有任何好處
總括來說,根據所有可用數據和信息,該產品所謂的益處,對患者或用戶造成了實質性欺騙,同時會致病或構成創傷,令用戶承擔不合理的重大風險。
FDA 禁止醫療產品常見嗎 ?
極罕見, 由 1983 至 2016 共 33 年間,祗有一種產品受禁 : 人工植髮。
2023 解禁了嗎 ?
近年來製造商試圖重新建立這些纖維絲的信譽,並將其引入歐洲、澳大利亞,香港等地。 該公司聲稱,之前與纖維絲相關的許多問題已經解決。 但 FDA 找不到任何公開的數據來支持這些說法,故仍未解禁。
美國 FDA 人工植髮禁令 1983 - 2023
美國 FDA 醫療產品禁令 2020
最新醫學指引
ISHRS
「 該產品對公共健康沒有任何好處,其對改善脫髮的優點,被誤導性地誇大。假髮纖維不會刺激頭髮生長,也不會有效掩蓋禿頭,而實際上可能會因植入異物,而導致嚴重的感染、皮膚病變,和對頭皮的傷害。本會認為現有標籤和廣告材料,直接或暗示該產品安全、有效,並且很少或根本沒有引起不適,是歪曲事實的誤導性聲明。 」
ISHRS 的聲明 ( Dec 2021 )
植髮專家
「 人工植髮是一個非常有爭議的話題。它的主要好處是捐髮區較差的患者可以成為使用來增髮。但有發炎和全身感染的顯著風險,需要去除纖維絲,並對感染進行全身性治療。可能會發生持續的發炎和異物反應,並導致纖維植入區域毀容,通常會出現凹坑疤痕。
有公司聲稱已經發明了新型改進的人造纖維,可以降低上述風險,並且已在一些國家使用,但作為皮膚科醫生,我們預計會看到大量異物反應持續發生。 」
Robin Unger, Ronald Shapiro. Hair Transplantation 6th Edition, Thieme 2023
人工植髮常見副作用
1. 反覆性細菌感染
2. 纖維的排斥和週期性損耗,需要經常更換
3. 持續性過敏反應,導致嚴重接觸性皮膚炎
4. 擔心可能致癌
5. 疤痕性脫髮
6. 肉芽腫性過敏 Granuloma
7. 囊腫形成 Cyst
8. 無法接受植髮
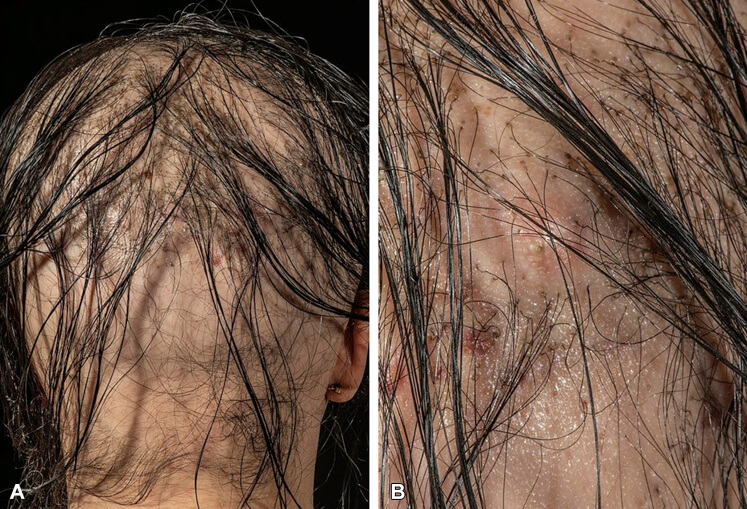
人工植髮副作用 : A,頭皮後部概覽。 B,同一區域的特寫。
一名患有先天性脫髮的 27 歲女性,在植入 9,000 根生物相容性纖維絲後,頭皮持續疼
痛和搔癢。 檢查發現多個、觸痛、帶有膿液的紅斑結節 ( 相片來源 )。
相關醫學文獻 ( 英文 )
連結
• Synthetic Hair Transplant, Dr Serkan Aygin
• Ban Hair Fiber and Synthetic Hair Implants, D'Vera Cohn
• Controversy: Synthetic Hairs and their Role in Hair Restoration, Int J Trichology. 2010 Jan-Jun; 2(1): 42–44
• Synthetic (Biofibre) Hair Transplant, 2019
• Synthetic hairs: Should they be used? Venkataram Mysore Consultant Dermatologist, India
• Staphylococcus lugdunensis and Trichophyton tonsurans Infection in Synthetic Hair Implants, Int J Trichology. 2017 Apr-Jun;9(2):82-86.
• Side effects after artificial hair implants: 2 case reports, JAAD Case Rep. 2020 Aug; 6(8): 740–742.
醫學文獻摘要 - 總結
Fiber implantation for pattern baldness. Review of complications in forty-one patients
C W Hanke, W F Bergfeld. J Am Acad Dermatol 1981 Mar;4(3):278-83.
Abstract
Forty-one patients who underwent one technic of fiber implantation for pattern
baldness were studied. Complications were frequent and included rejection of fibers,
infection, facial swelling, pain, pruritus, and scarring. In addition, patients incurred risks
of carcinogenesis from deeply embedded bits of fiber and also loss of their own natural
hair. Fiber implantation is an unsuccessful method of hair restoration, and its use has
been discouraged by state and federal agencies.
Complications of fiber implantation for baldness
C W Hanke, R I Ceilley, W F Bergfeld, Case Reports Am Fam Physician 1981 Jul;24(1):115-8.
Abstract
Several methods of fiber implantation have been used to reduce pattern baldness, and
similar results have been reported: fiber breakage, foreign-body reaction, infection and
scarring. Since one method or another tends to predominate in different geographic
areas, the physician often is unable to compare the results of the various methods. The
two patients described in this article underwent different methods of fiber implantation;
serious complications occurred in both. Fiber implantation is an unsafe technique and
must be discouraged.
Hair Implant Complications
C W Hanke, A L Norins, J G Pantzer Jr, J E Bennett. Case Reports JAMA 1981 Apr 3;245(13):1344-5.
Abstract
Four men who underwent hair implantation for pattern baldness were treated for
complications such as infection, foreign-body reaction, pruritus, and scarring. The
complications were similar to those reported with synthetic modacrylic fiber implants
that have been used for the same purpose. Although we believe this is the first article to
report complications from hair implants, the illogical basis of the procedure suggests
that complications will occur in many unsuspecting patients who undergo hair
implantation.
Fiber Implantation For Pattern Baldness
C W Hanke, W F Bergfeld. Case Reports JAMA 1979 Jan 12;241(2):146-8.
Abstract
Examination of 20 patients who had fiber implantation for the treatment of pattern
baldness showed that nearly all the fibers had fallen out by ten weeks. Complications
observed were facial swelling, infection, foreign-body granulomas, scarring, and
permanent hair loss. Scanning electron microscopy identified the fibers as modacrylic
fibers. The complications, high monetary cost, and ultimate futility of fiber implantation
make it an unacceptable procedure.
Dangers of synthetic fiber implantation for male pattern baldness
R S Schwartz, T F Downham 2nd Case Reports Cutis 1980 May;25(5):491-2.
Abstract
A case report is presented herein of synthetic fiber implantation for male pattern
baldness with secondary foreign body granuloma formation and persistent infection of
the scalp.
Therapy and histopathology of complications from synthetic fiber implants for hair replacement. A presentation of one hundred cases
M I Lepaw. Case Reports J Am Acad Dermatol. 1980 Aug;3(2):195-204.
Abstract
One hundred cases of postoperative complications due to inserting synthetic wig fibers
into the scalps of patients seeking to remedy their patterned alopecia were studied.
Modes of therapy to remedy these problems were evaluated. The best results were
obtained by removing the offending material. Histopathology of the lesions is discussed.
The severity of the complications of synthetic hair implants to remedy baldness makes
the implantation of fibers into the scalp a dangerous, futile, and expensive approach,
which should be abandoned immediately. Cosmetic reconstruction should be deferred
at least 3 to 6 months after the last fiber is removed.
Cutaneous complications of artificial hair implantation: a pathological study Case Reports Dermatology
A M Peluso 1, P A Fanti, M Monti, F Bardazzi, A Tosti. 1992;184(2):129-32.
Abstract
Five patients who developed severe cutaneous complications after artificial hair
implantation were subjected to a scalp biopsy. The pathology showed the presence of
hyperplastic epidermal proliferations that produced infundibulum-like structures
around the implanted fibers in the superficial dermis. A dense acute inflammatory
infiltrate surrounded the artificial fibers in the superficial dermis. In the deep dermis a
granulomatous infiltrate was present whereas in the hypodermis the inflammatory
infiltrate was sparse and the fibers were embedded in fibroplasia. The pathology of a
patient who did not present any skin inflammation after artificial hair implantation
showed similar pathological features but the absence of acute inflammation suggesting
that bacterial infections play a major role in the development of the cutaneous
complications of hair implantation. Since definitive treatment of the infections is
ineffective until the fibers are removed from the scalp, surgical treatment was required
in 2 of our patients
